does co2 have polar or nonpolar bonds Polar vs nonpolar molecules- definition, 7 key differences, examples
Hey there! Today, let’s talk about polar and nonpolar molecules. You may have heard these terms before, but what do they actually mean? To start off, let’s define what a molecule is. A molecule is a group of atoms that are chemically bonded together. Polar and nonpolar molecules differ in the way their atoms are bonded together and how they interact with other molecules. So, what makes a molecule polar or nonpolar? It all comes down to the distribution of electric charge within the molecule. In a polar molecule, the electric charge is not distributed evenly, resulting in a partial positive and negative charge on different sides of the molecule. In contrast, a nonpolar molecule has an even distribution of electric charge, resulting in no partial charges on any side of the molecule. One example of a polar molecule is water (H2O). The oxygen atom has a higher electronegativity than the hydrogen atoms, meaning it attracts electrons more strongly. As a result, the electrons spend more time around the oxygen atom, giving it a partial negative charge, while the hydrogen atoms have a partial positive charge. This uneven charge distribution allows water molecules to interact with other polar molecules, such as other water molecules, or ions, like sodium and chloride in salt. On the other hand, an example of a nonpolar molecule is methane (CH4). In CH4, the carbon atom shares electrons equally with the hydrogen atoms, resulting in no partial charges. This even distribution of charge means that methane molecules don’t interact with other polar molecules very well. Now that we know what makes a molecule polar or nonpolar, let’s take a look at some key differences between the two. 1. Solubility: Polar molecules are soluble in polar solvents, such as water, but not in nonpolar solvents, such as oils. Nonpolar molecules are soluble in nonpolar solvents, but not in polar solvents. 2. Boiling and melting points: Polar molecules have higher boiling and melting points than nonpolar molecules, as the partial charges create stronger intermolecular forces that hold the molecules together. 3. Reactivity: Polar molecules tend to be more reactive than nonpolar molecules, as they can participate in more types of chemical reactions due to the partial charges on the molecule. In conclusion, understanding the difference between polar and nonpolar molecules can help us understand the properties and behaviors of different substances. Next time you come across a molecule, try to determine if it’s polar or nonpolar and think about how that affects its interactions with other molecules. Thanks for reading! Check out the images below for a visual representation.
Polar Molecules
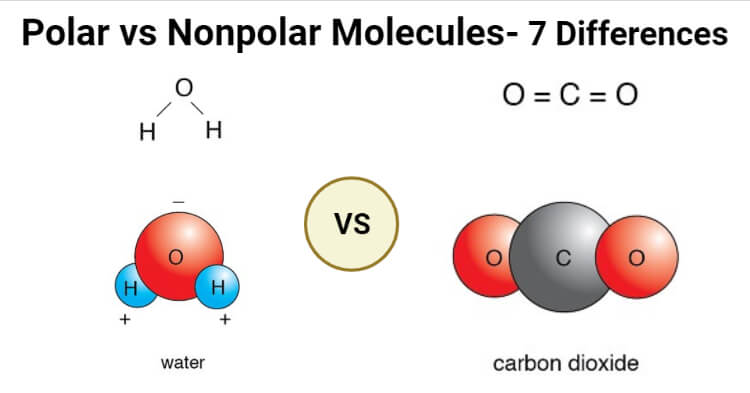 A visual representation of the difference between polar and nonpolar molecules. Image source: The Chemistry Notes.
A visual representation of the difference between polar and nonpolar molecules. Image source: The Chemistry Notes.
Nonpolar Molecules
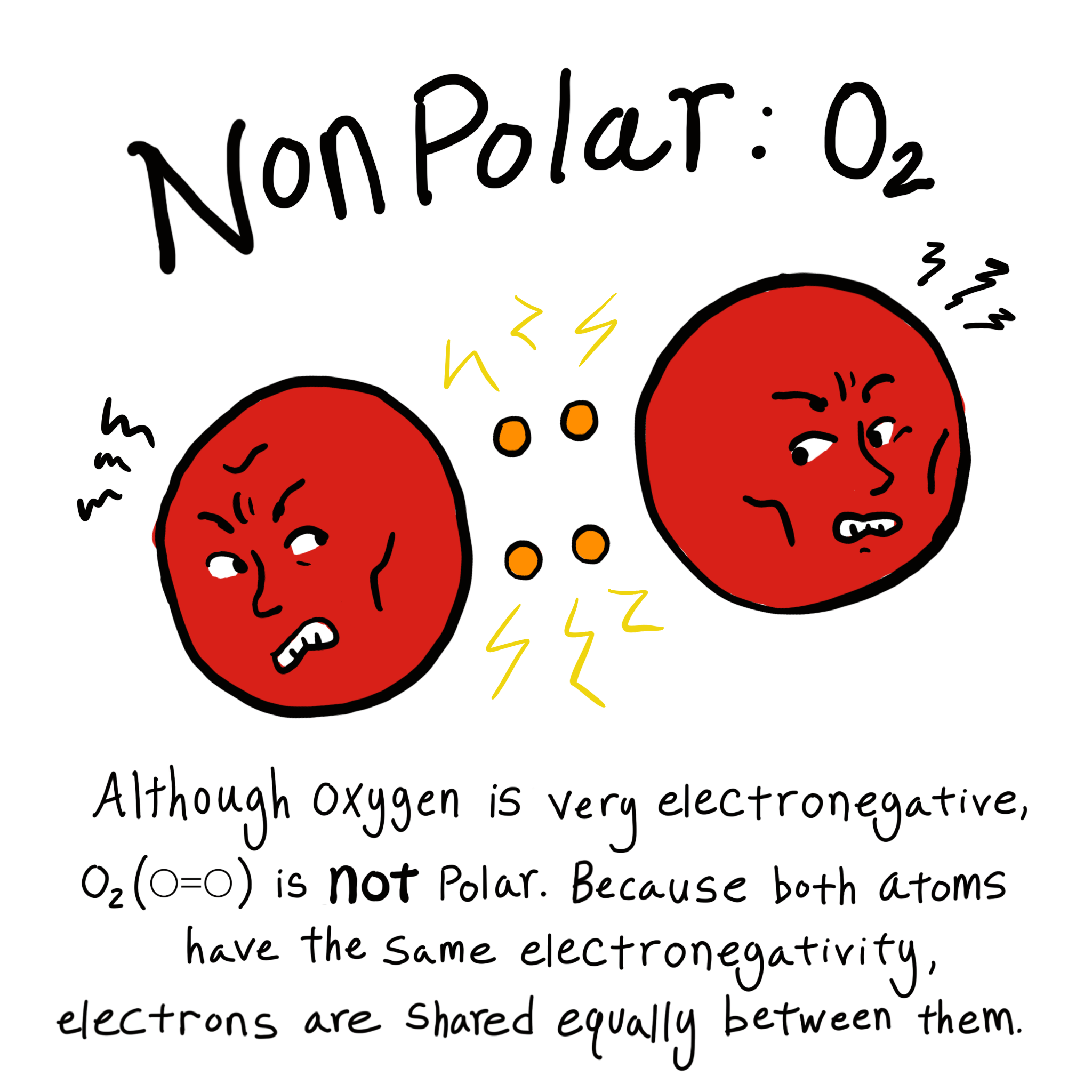 An example of a nonpolar molecule, CH4. Image source: Simple English Wikipedia.
An example of a nonpolar molecule, CH4. Image source: Simple English Wikipedia.
If you are searching about Polar vs Nonpolar Molecules- Definition, 7 Key Differences, Examples you’ve came to the right page. We have 5 Pics about Polar vs Nonpolar Molecules- Definition, 7 Key Differences, Examples like JensentuHuang, Is Carbon Dioxide (CO2) Polar Or Nonpolar? » Science ABC and also JensentuHuang. Here it is:
Polar Vs Nonpolar Molecules- Definition, 7 Key Differences, Examples
 thechemistrynotes.comnonpolar molecules molecule differences substance atoms
thechemistrynotes.comnonpolar molecules molecule differences substance atoms
JensentuHuang
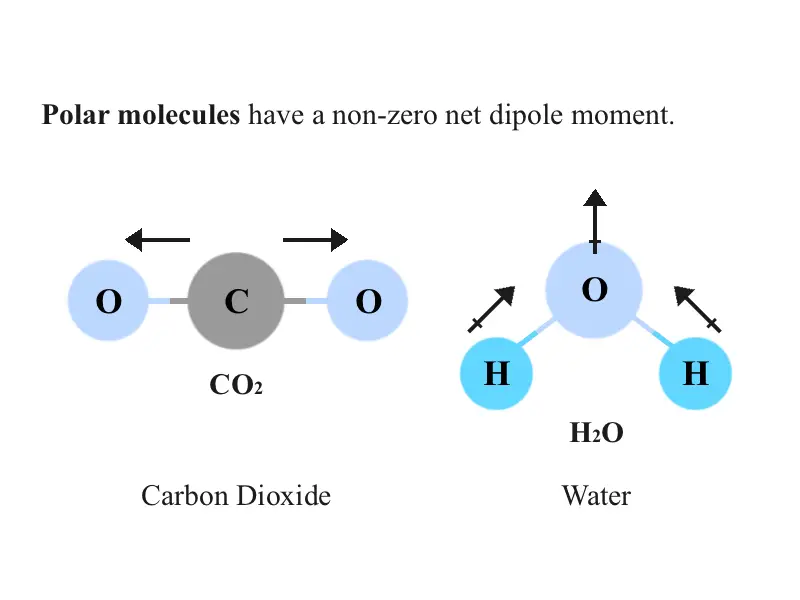 jensentuhuang.blogspot.comPolar Vs. Nonpolar
jensentuhuang.blogspot.comPolar Vs. Nonpolar
 users.stlcc.edupolar covalent bond bonds ionic nonpolar non compounds chemistry molecule if electronegativity difference determine bonding example between meant diagrams chemical
users.stlcc.edupolar covalent bond bonds ionic nonpolar non compounds chemistry molecule if electronegativity difference determine bonding example between meant diagrams chemical
Is Carbon Dioxide (CO2) Polar Or Nonpolar? » Science ABC
 www.scienceabc.comdioxide nonpolar bonds molecule bond atoms vidalondon mugeek atom
www.scienceabc.comdioxide nonpolar bonds molecule bond atoms vidalondon mugeek atom
Ch4 Polar Or Nonpolar Covalent Bond - Covalent Bond - Simple English
 rhiannond-decree.blogspot.comnonpolar covalent bonds molecules decree electrons
rhiannond-decree.blogspot.comnonpolar covalent bonds molecules decree electrons
Polar vs. nonpolar. Polar covalent bond bonds ionic nonpolar non compounds chemistry molecule if electronegativity difference determine bonding example between meant diagrams chemical. Ch4 polar or nonpolar covalent bond